Incidental findings: Results that arise that are outside the original purpose for which the test or procedure was conducted.
Overview
As imaging technology continues to advance, anomalies are increasingly more detectable, making incidental findings more commonplace. Recent systematic reviews (1) noted the mention of these findings in radiology reports is as high as 31 percent. However, proper communication and follow-up of incidental findings occur in about one in three occurrences. With early detection being one of the most effective tools to beat cancer, proper management of incidental findings has significant implications for patient care and risk mitigation for healthcare systems.
The State of Incidental Findings
Abdominal CT scans produce incidental findings in an estimated 30 to 25 percent of scans in a study of CT scans of patients at a trauma center, 43 percent of patients had at least one incidental finding (2).
An estimated 4.8 million people had chest CT scans in the USA per a 2015 study, in which 63,000 (1.3% of all chest CT scans) revealed malignant nodules in the lung parenchyma (1).
A 2014 study published in the Journal of the American College of Radiology found that approximately 1 in 10 CT pulmonary angiographic studies ordered in the emergency department indicated an incidental pulmonary nodule (in a review of 1,000 studies). Follow-up of these incidentals occurred 29% of the time, with no follow up when the nodules were mentioned solely in the findings section of reports (1).
In another study, the number of incidental cancer diagnosis was 4 percent among cancer patients – indicating 1 in 25 patients have been diagnosed with cancer due to incidental findings occurring while performing imaging or other tests (3).
Risks of Incidental Findings
Failures to make a timely diagnosis of lung cancer often falls to either the family physician or radiologist. This is generally caused by misreading a chest CT scan; the ordering physician fails to see an abnormality in the report or recognize the significance of incidental findings, or when recognized, the finding does not receive proper attention and communication to the patient. A nationwide U.S. survey by Whang et al (4), identified error in diagnosis as the most common cause for radiology malpractice suits, followed by inadequate communication of important findings to the referring physician or clinician who is caring for the patient, and failure to recommend additional testing.
Oftentimes, radiologists detect such unexpected abnormalities in imaging studies done for other purposes, but health systems frequently lack the processes to ensure that patients and providers follow through. Experts estimate that compliance for follow-up ranges from 29% to 77% (1).
Failing to identify cancer and potential symptoms or communicate such findings, can deprive patients of life-saving treatment options. Also, to a patient experiencing several health problems, including premature death, health systems can be held responsible for significant monetary reparations to cover the costs of further treatment, lost wages, and pain and suffering associated with an untimely or avoidable death.
- 2017, Illinois $7,500,000 Settlement: Male patient had sought care at the University of Illinois for possible venous stenosis in his kidney, which included a CT scan of his abdomen and pelvis. The radiologist spotted a mass on his lungs and recommended additional tests, but did not share that info with the patient. The patient returned 18 months later after experiencing pain and swelling in his shoulder and chest. A new CT scan revealed lung cancer that had metastasized after a few months of treatment and the patient died at age 63. The Chicago-based hospital settled for $7.5 million over the delayed lung cancer diagnosis.
- 2017, Virginia $1,470,000 Settlement: 77-year-old male developed a persistent cough and underwent a Chest x-ray at urgent care, which was interpreted as showing a density in his left lung. The patient was referred to a pulmonologist. A second x-ray was ordered and the radiologist read it as normal, failing to compare the two films. The following year the patient was diagnosed with Stage IV lung cancer with metastasis to his brain and bones and died four months later. The jury found both the pulmonologist and radiologist were jointly and severally liable for negligence in failing to timely diagnose lung cancer and the parties settled for $1.47 million.
- 2018, Massachusetts $1,000,000 Settlement: Female patient in her early 70s underwent a CT scan as part of her treatment for cardiac disease. The radiology report identified a potentially suspicious mass in the patient’s lung and recommended a 6-month follow up CT scan. The report was sent to the primary care physician, but no 6-month follow up (or any follow up) was performed. By the time the lung cancer was diagnosed, it had spread to her liver and bones and she died shortly after. The primary care doctor was sued for negligently failing to follow up with the 6 month CT scan and the case was settled for $1 million.
- 2017, Maryland $461,862 Verdict: 79-year-old male with a history of smoking underwent chest CT scan as a part of a lung cancer screening clinic at GBMC. The radiologist identified two “nodular areas” in the patient’s lungs and submitted findings to the patient’s primary care physician, but without a clear diagnosis or recommendation. The primary care physician did not perform any follow-up exam. Two years following the CT scan the patient was diagnosed with Stage IV lung cancer and died soon after. A jury found the primary care doctor was negligent and awarded the estate $461,862.
While federal and state statutes may not directly address the physician’s duty to return incidental or secondary findings to patients, medical malpractice law has interpreted the potential legal liability a clinician might face for failing to identify and disclose such findings.
Recommendations on Incidental Findings
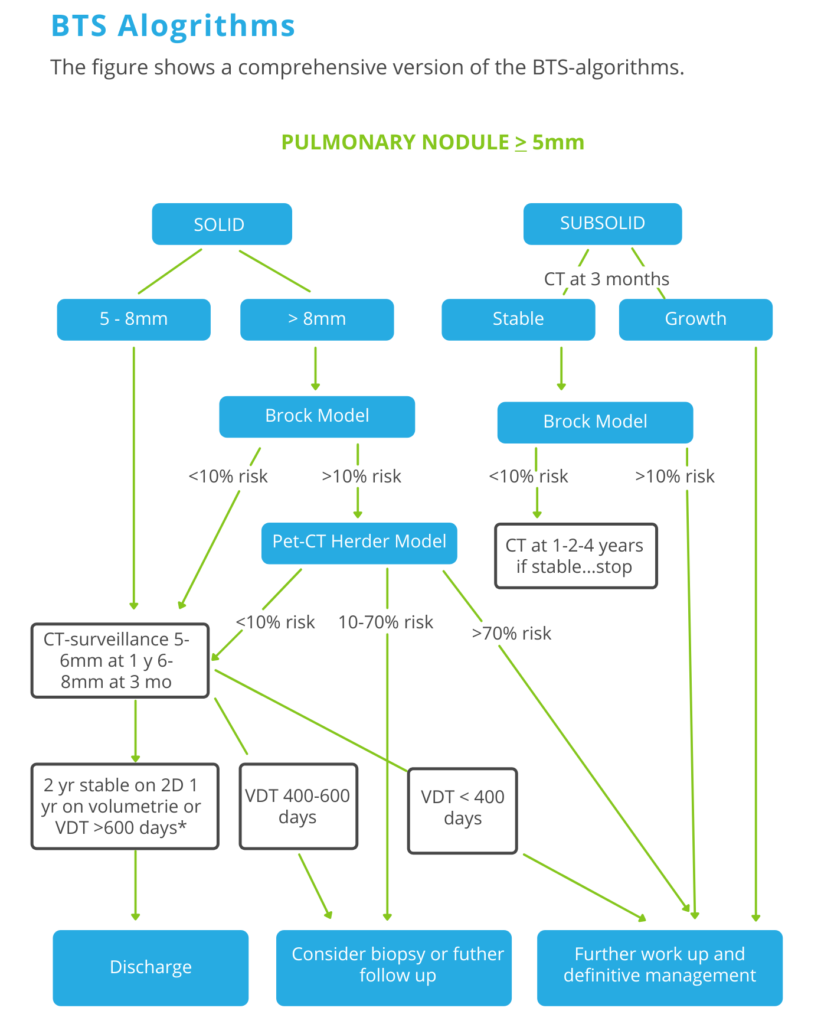
The Brock model, also known as the PanCan model, is a multivariable model that estimates the risk that a pulmonary nodule on a CT scan is lung cancer. It has been recommended by the British Thoracic Society guidelines for pulmonary nodules.
Step 1
No, follow up for nodules < 5mm and typically benign lesions with benign calcifications like hamartomas and perifissural nodules.
Step 2
Only lesions of 5mm or more required follow up. Divide lesions into solid and subsolid (ground glass or part solid).
Step 3
Use the Brock Model application to assess the risk of malignancy for solid lesions >8mm and subsolid lesions that are stable during the 3-month follow-up.
Step 4
Use the Herder model when you perform a PET-CT.
Follow-up takes 1 year if volumetry is used, while manual 2D-measurements warrant a 2 year follow-up period.
Nodules that show volume change less than 25% should be regarded stable and discharged after the indicated follow-up interval.
Consider discharge only if VDT >600 days is calculated using volumetry.
When there is previous imaging, determine the risk of lung cancer based on the volume doubling time.
Managing Incidental Findings
Advanced technologies like Thynk Health’s Nodule Nav solution can improve follow-up adherence for LDCT from 15-20% to 60% or higher. Advanced nodule surveillance and management solutions like Nodule Nav can improve follow-up from 30% to near 80%.
How does Thynk Health’s solution work? The innovative technology unites recurrent neural architectures training for each organization, allowing the solution’s advanced artificial intelligence to learn each provider’s unique style of reporting and processes extracted data through expert-designed rule systems. This allows the Nodule Nav solution to process radiology reports and identify entities (such as finding, location, size) and the relationship of the entities in radiology reports. The Nodule Nav solution works on a general recurrent neural network (RNN), in which models are evaluated in their ability to identify entities (such as finding, location, size) and the relationship of the entities in radiology reports.
Secondly, Thynk Health evaluates the performance of the combination of an expert logic layer in addition to an RNN layer. This combination drives accuracy or overall F1 scores to levels not otherwise seen in the industry and allows for a very automated healthcare software system.
The clinical team can automatically track nodule changes within each appointment, flag nodules for follow-up, and recommend next steps (customized to your healthcare system’s policies). All of this is accomplished via artificial intelligence, allowing radiologists to keep traditional workflows in place.
The Nodule Nav solution’s rapid deployment mechanism and the ability to extend to all incidental findings in multiple organizations are proprietary to Thynk Health and is only achieved through customized interfaces that allow in-house clinical experts to rapidly review reporting style discrepancies among radiologists.
References
- Gould MK Tang T Liu IL et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 192(10), 1208–1214 (2015).Crossref, Medline, Google Scholar
- Anticipate and Communicate Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct-to-Consumer Contexts, Link
- Koo MM, Rubin G, Mcphail S, Lyratzopoulos G. Incidentally diagnosed cancer and commonly preceding clinical scenarios: a cross-sectional descriptive analysis of English audit data. BMJ Open9(9), e028362 (2019).Crossref, Medline, Google Scholar
- Whang JS, Baker SR, Patel R, Luk L, Castro A 3rd. The causes of medical malpractice suits against radiologists in the United States. Radiology 2013;266(2):548–554. Link, Google Scholar